
英语原文共 39 页,剩余内容已隐藏,支付完成后下载完整资料
|
英文原文 |
译文 |
|
6 FORMATION MECHANISM 0F NITROGEN OXIDES |
6 氮氧化物的形成机制 |
|
Formation mechanism of oxides of nitrogen has been a topic of intensive research for many decades.The importance of this mechanism stems from the fact that nitrogen oxides are one of the principal contaminants emitted by combustion processes. Oxides of nitrogen damage the environment severely; thus, government agencies are passing stringent laws to control the emissions of pollutants. An in-depth understanding of the mechanism of formation of the oxides of nitrogen is essential when automotive and other industrial devices are designed. Suitable schemes must be developed for controlling the amount of NOx generated from combustion products of various engines and other energy-conversion systems. Another motivation for the study of NOx formation mechanism arises from the fact that energetic materials, such as explosives, always contain nitrogen com- pounds, and nitrogen oxides are always present in their combustion products. Therefore, knowledge of the N0x formation mechanism is essential for under-standing combustion of energetic materials. |
氮的氧化物的形成机制已经是这几十年的一个研究热点。这一机制的重要性在于,氮氧化物是燃烧过程的主要排放污染物。氧化物对环境造成了严重危害,因此,政府机构通过严格的法律来控制污染物的排放量。在汽车和其他工业装置的设计过程中,对氮氧化物的形成机理的深入了解是必要的。必须制定合适的计划,以控制来自各种发动机和其他能量转换系统中燃烧产生的氮氧化物的量。对氮氧化物形成机制研究的另一个动机来自于这样一个事实,即含能材料,如炸药,他们的燃烧产品中总含有氮元素,和氮氧化物。因此,出于含能材料的燃烧,对尾气的形成机制的知识的了解是必不可少的。 |
|
The first major work on the kinetics of NOx was performed by Zeldovich in 1946, who postulated the thermal NO mechanism, now known as the Zeldovich NO mechanism. Later on, Fenimore142 in1979 proposed the prompt NO mechanism to explain the additional NO being produced over and above the Ze1dovich thermal NO in hydrocarbon flames. Recently, researchers identified some additional routes through which NO and other oxides of nitrogen are formed. These different routes are categorized below: |
对氮氧化合物的动力学的第一个主要工作是Zeldovich在1946年进行的,而热氮氧机制,现在则被称为Zeldovich氮氧机制。后来,Fenimore在1946年提出的快捷氮氧机制来解释产生在烃火焰中不超过Ze1dovich 热氮氧的额外NO。最近,研究人员通过氮和其他氧化物的形成发现了一些额外的路线。这些不同的路线分以下: |
|
|
|
Their individual mechanisms are described in the following sections. |
它们个人的机制在以下各节描述。 |
|
16.1 Thermal NO Mechanism(Zeldovich Mechanism) |
16.1 热氮氧机制(Zeldovich 机制) |
|
In the combustion of clean fuels(which do not contain nitrogen compounds) with air(which contains atmospheric N2) N0 is formed mainly by the Ze1dovich mechanism |
在清洁燃料的燃烧(不含氮的化合物)与空气(包含大气中氮气)NO主要是由Ze1dovich 机制形成。 |
|
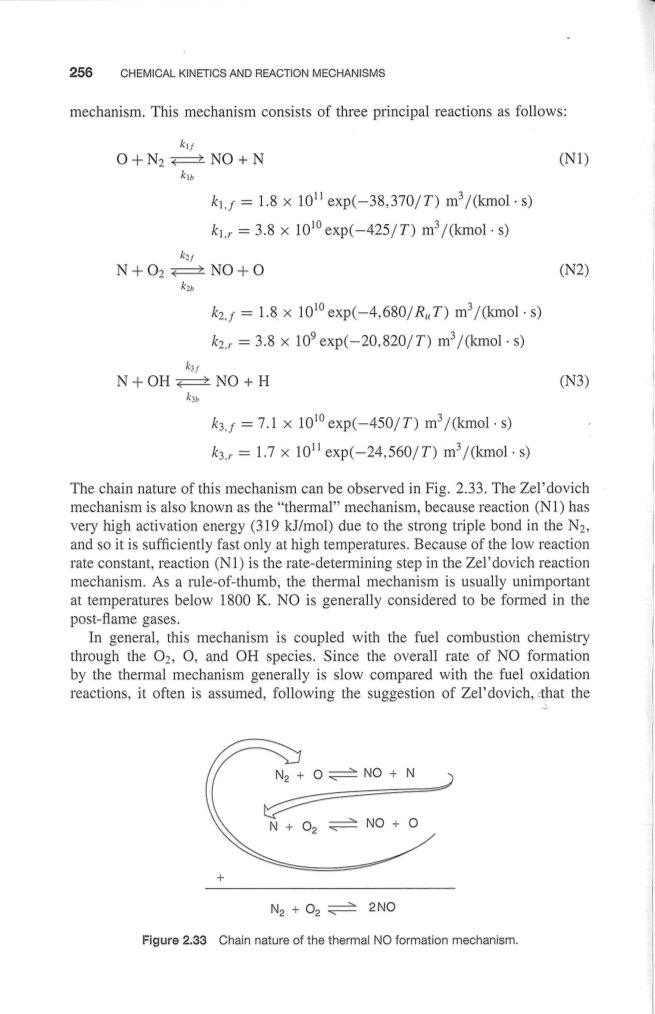
This mechanism consists of three principal reactions as follows: |
该机制包括三个主要的反应如下: |
|
The chain nature of this mechanism can be observed in Fig. 2.33. The Zeldovich mechanism is also known as the“thermal” mechanism, because reaction(N1) has very high activation energy(319 kJ/mol) due to the strong triple bond in the N2, and so it is sufficiently fast only at high temperatures. Because of the low reaction rate constant. reaction(N1) is the rate-determining step in the Zeldovich reaction mechanism. As a rule-of-thumb, the thermal mechanism is usually unimportant at temperatures below 1800 K. N0 is generally considered to be formed in the post-flame gases. |
这一机制链的性质可以在图2.33中观察到的。该Zeldovich 机制也被称为“热”的机制,因为反应(N1)具有很高的活化能(319 kJ/mol)由于在N2的强三键,所以在高温下,它是足够快。这是由于低的反应速率常数。反应(N1)是在Zeldovich 反应机制的速度控制步骤。作为一个经验法则,热机制在温度低于1800K时通常是不重要的。氮氧被认为是在后火焰气体形成的。 |
|
In general, this mechanism is coupled with the fuel combustion chemistry through the 02, 0, and 0H species. Since the overall rate of N0 formation by the thermal mechanism generally is slow compared with the fuel oxidation reactions, it often is assumed, following the suggestion of Zeldovich,that the N0 formation reactions can be decoupled from the fuel oxidation process. The concentration of N0 cannot be predicted well by considering it to be in equilibrium in reaction(N1), because the reaction(N1) is so slow that equilibrium is reached only for times that are much longer than typical; residence times in the high-temperature range. |
总的来说,这一机制是通过氧气,氧和氢氧,与燃料燃烧化学联合来实现的。与燃料的氧化反应相比,由热机制形成整体速度的氮氧反应是缓慢的,但这往往是假设;根据Zeldovich的建议,氮氧生成反应可以从燃料的氧化过程分离。氮氧浓度在反应(N1)中认为是平衡的,因为反应(N1)是如此的缓慢,以致于达到平衡的时间时,在高温范围比典型的停留时间更长。 |
|
Quasi-steady-state assumption for N atom can be used to simplify the rate expression of N0 formation. If the assumption that the N0 concentrations are much less than the equilibrium values is made, the reverse reactions can be neglected. These assumptions greatly simplify the calculation of N0 formation rate. Using reactions(N1), (N2), and(N3), Assuming N atoms to be in quasi steady state[because of fast reactions(N2) and(N3)], dC1v/df= 0; thus, Solving Eq. (2-180) for CA and substituting it into Eq. (2-179), we get |
剩余内容已隐藏,支付完成后下载完整资料 资料编号:[147467],资料为PDF文档或Word文档,PDF文档可免费转换为Word |
以上是毕业论文外文翻译,课题毕业论文、任务书、文献综述、开题报告、程序设计、图纸设计等资料可联系客服协助查找。


